Lithium steel batteries with strong electrolytes are a promising generation because of their light-weight, non-flammable nature, prime power density, and instant recharging skill. Alternatively, their building has been hindered via the problem of short-circuiting and failure. Scientists at Stanford College and SLAC Nationwide Accelerator Laboratory declare to have resolved this thriller.
New lithium steel batteries with strong electrolytes are light-weight, inflammable, pack numerous power, and may also be recharged in no time, however they have got been gradual to expand because of mysterious short-circuiting and failure. Now, researchers at Stanford College and SLAC Nationwide Accelerator Laboratory say they have got solved the thriller.
It comes down to worry – mechanical tension to be extra actual – particularly all over potent recharging.
“Simply modest indentation, bending or twisting of the batteries could cause nanoscopic fissures within the fabrics to open and lithium to interfere into the cast electrolyte inflicting it to quick circuit,” defined senior creator William Chueh, an affiliate professor of fabrics science and engineering within the Faculty of Engineering, and of power sciences and engineering within the new Stanford Doerr Faculty of Sustainability.
“Even mud or different impurities offered in production can generate sufficient tension to motive failure,” mentioned Chueh, who directed the analysis with Wendy Gu, an assistant professor of mechanical engineering.

This artist’s rendition presentations one probe bending from implemented force, inflicting a fracture within the strong electrolyte, which is filling with lithium. At the proper, the probe isn’t urgent in opposition to the electrolyte and the lithium plates at the ceramic floor, as desired. Credit score: Cube3D
The issue of failing strong electrolytes isn’t new and lots of have studied the phenomenon. Theories abound as to what precisely is the motive. Some say the unintentional waft of electrons is responsible, whilst others level to chemistry. But others theorize other forces are at play.
In a learn about printed as of late (January 30) within the magazine Nature Power, co-lead authors Geoff McConohy, Xin Xu, and Teng Cui provide an explanation for in rigorous, statistically important experiments how nanoscale defects and mechanical tension motive strong electrolytes to fail. Scientists around the globe looking to expand new, strong electrolyte rechargeable batteries can design round the issue and even flip the invention to their merit, as a lot of this Stanford staff is now researching. Power-dense, fast-charging, non-flammable lithium steel batteries that remaining a very long time may triumph over the principle limitations to the in style use of electrical cars, amongst a lot of different advantages.
Statistical importance
Lots of as of late’s main strong electrolytes are ceramic. They permit instant shipping of lithium ions and bodily separate the 2 electrodes that retailer power. Most significantly, they’re fireproof. However, like ceramics in our houses, they are able to expand tiny cracks on their floor.
The researchers demonstrated thru greater than 60 experiments that ceramics are regularly imbued with nanoscopic cracks, dents, and fissures, many lower than 20 nanometers large. (A sheet of paper is ready 100,000 nanometers thick.) Throughout instant charging, Chueh and staff say, those inherent fractures open, permitting lithium to interfere.
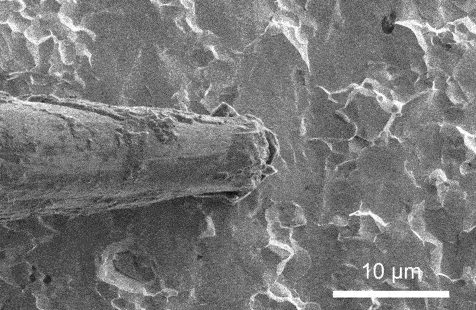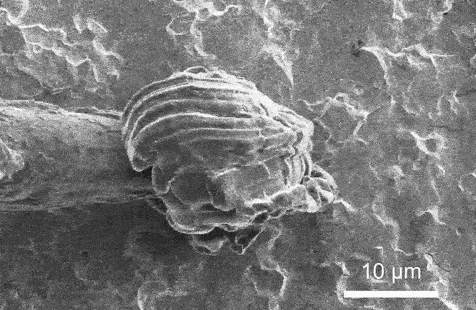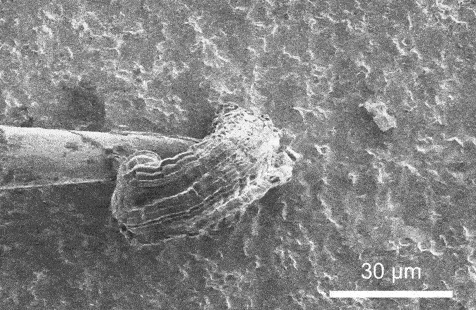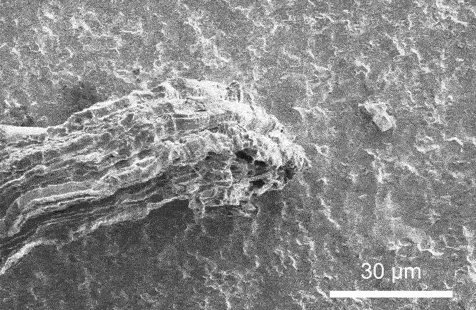
A scanning electron microscopy video that presentations lithium plating because it takes position on a strong electrolyte. Credit score: Xin Xu, Geoff McConohy and Wenfang Shi
In each and every experiment, the researchers implemented {an electrical} probe to a strong electrolyte, making a miniature battery, and used an electron microscope to watch instant charging in actual time. Therefore, they used an ion beam as a scalpel to know why the lithium collects at the floor of the ceramic in some places, as desired, whilst in different spots it starts to burrow, deeper and deeper, till the lithium bridges around the strong electrolyte, growing a brief circuit.
The variation is force. When {the electrical} probe simply touches the skin of the electrolyte, lithium gathers fantastically atop the electrolyte even if the battery is charged in lower than one minute. Alternatively, when the probe presses into the ceramic electrolyte, mimicking the mechanical stresses of indentation, bending, and twisting, it’s extra possible that the battery quick circuits.
Principle into observe
An actual-world solid-state battery is manufactured from layers upon layers of cathode-electrolyte-anode sheets stacked one atop any other. The electrolyte’s position is to bodily separate the cathode from the anode, but permit lithium ions to trip freely between the 2. If cathode and anode contact or are hooked up electrically by any means, as via a tunnel of steel lithium, a brief circuit happens.
As Chueh and staff display, even a refined bend, slight twist, or speck of mud stuck between the electrolyte and the lithium anode will motive imperceptible crevices.
“Given the chance to burrow into the electrolyte, the lithium will ultimately snake its manner thru, connecting the cathode and anode,” mentioned McConohy, who finished his doctorate remaining yr operating in Chueh’s lab and now works in business. “When that occurs, the battery fails.”

Co-lead authors of the brand new learn about, from left, Xin Yu, Teng Cui and Geoff McConohy sitting in entrance of the centered ion beam/scanning electron microscope used for this analysis. Credit score: Xin Xu
The brand new figuring out was once demonstrated time and again, the researchers mentioned. They recorded video of the method the use of scanning electron microscopes – the exact same microscopes that had been not able to peer the nascent fissures within the natural untested electrolyte.
It’s slightly like the best way a pothole seems in differently easiest pavement, Xu defined. Via rain and snow, automobile tires pound water into the tiny, pre-existing imperfections within the pavement generating ever-widening cracks that develop over the years.
“Lithium is in truth a cushy subject matter, however, just like the water within the pothole analogy, all it takes is force to widen the space and motive a failure,” mentioned Xu, a postdoctoral student in Chueh’s lab.
With their new figuring out in hand, Chueh’s staff is taking a look at techniques to make use of those exact same mechanical forces deliberately to reinforce the fabric all over production, just like a blacksmith anneals a blade all over manufacturing. They’re additionally taking a look at techniques to coat the electrolyte floor to forestall cracks or restore them in the event that they emerge.
“Those enhancements all get started with a unmarried query: Why?,” mentioned Cui, a postdoctoral student in Gu’s lab. “We’re engineers. A very powerful factor we will do is to determine why one thing is occurring. When we know that, we will support issues.”
Reference: “Mechanical legislation of lithium intrusion likelihood in garnet strong electrolytes” via Geoff McConohy, Xin Xu, Teng Cui, Edward Barks, Sunny Wang, Emma Kaeli, Celeste Melamed, X. Wendy Gu and William C. Chueh, 30 January 2023, Nature Power.
DOI: 10.1038/s41560-022-01186-4
Chueh could also be a senior fellow on the Precourt Institute for Power at Stanford, and a college scientist at SLAC. Co-authors of the learn about no longer discussed above are Stanford PhD scholars Edward Barks, Sunny Wang, and Emma Kaeli, and postdoctoral student Celeste Melamed.
Investment: Samsung Complicated Institute of Generation, Automobile Applied sciences Place of business, Stanford StorageX Initiative
Supply By means of https://scitechdaily.com/stanford-breakthrough-paves-way-next-generation-lithium-metal-batteries-that-charge-very-quickly/